Recently, Professor Taicheng An's team from the School of Environmental Science and Engineering and the Institute of Environmental Health and Pollution Control, in collaboration with Professor Joseph S. Francisco's team from the University of Pennsylvania, USA, has made significant progress in theoretical chemical calculations of the formation of nitrochloridic acid (ClNO2) at the air-liquid interface and its chlorine radical generating mechanism. The research results, titled "Distinctive heterogeneous reaction mechanism of ClNO2 on the air-water surface containing Cl", were published on 9 October 2023 in the Journal of the American Chemical Society, 2023, 145: 22649-22658. The first author of the paper is Associate Professor Zhang Weina, and the corresponding authors are Professor An Taicheng and Professor Joseph S. Francisco, and Guangdong University of Technology is the first unit of the paper.
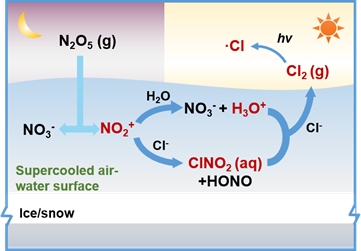
Recent studies on tropospheric atmospheric chlorine chemistry have found that chlorine radicals are highly oxidative and can rapidly oxidise hydrocarbon organic pollutants in the troposphere, thus affecting the atmospheric concentrations of hydroxyl radicals, peroxyl radicals and alkoxyl radicals, and are therefore closely related to the occurrence of tropospheric ozone pollution, which ultimately has important implications for atmospheric oxidability in the troposphere, the generation of secondary pollutants in the winter, and the health and protection of human ecosystems. ClNO2 is an important source of chlorine radicals and reactive nitrogen compounds, and therefore affects atmospheric oxidability and plays an important role in the global nitrogen and chloride cycles. In cold regions, ClNO2 mainly originates from the non-homogeneous reaction of nitrogen pentoxide (N2O5) on the surface of chloride-containing ice/snow. Therefore, scientists from China and the United States combined multiple theoretical chemical algorithms to investigate the microkinetic transformation process of ClNO2 at the supercooled gas-liquid interface and its contribution mechanism to the formation of chlorine radicals, revealed the non-homogeneous chemical reaction mechanism between ClNO2 and chloride ions at the acidified gas-liquid interface, and proposed that the acidic ions generated by N2O5 hydrolysis are the prerequisites for its rapid production of reactive chlorine. The results not only help to correctly understand the source of atmospheric chlorine radicals in cold regions, but also provide basic data for the contribution of atmospheric chlorine chemical reactions to tropospheric atmospheric oxidability, secondary pollutant formation and ozone production.
Original link: https://pubs.acs.org/doi/10.1021/jacs.3c07843